Total Talus ReplacementParagon 28® Patient Specific Talus Spacer
Paragon 28® Patient Specific Talus Spacer
Paragon 28® Patient Specific Talus Spacer
PRODUCT DESCRIPTION
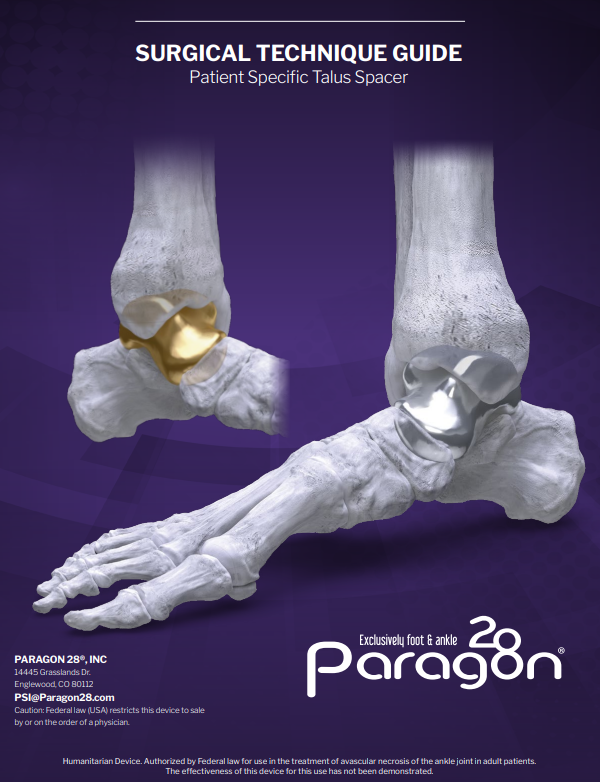
The Paragon 28® Patient Specific Talus Spacer is an alternative treatment option to fusion and amputation and requires talus replacement surgery. During this procedure the native talus bone is removed and replaced with a 3D printed replica. This is considered a joint-sparing procedure, as it allows the patient to maintain motion of his/her ankle joint.
The Paragon 28® Patient Specific Talus Spacer is an additively manufactured Paragon 28® Patient Specific Talus Implant.
- The first and only FDA approved total talus implant on the market available in multiple materials
- Indicated for Avascular Necrosis of the ankle
- Provides pain relief and preserves motion of the ankle joint
- Alternative to fusion or amputation
- The Paragon 28® Patient Specific Talus Spacer is an additively manufactured, or 3D printed, patient specific implant that is designed and made individually for each patient using CT image data.
The Paragon 28® Patient Specific Talus Spacer is indicated for avascular necrosis of the ankle joint. The anatomical landmarks necessary for the design and creation of the Paragon 28® Patient Specific Talus Spacer must be present and identifiable on computed tomography (CT) scan.
Xiomalis' Patient Story
The Paragon 28 Patient Specific Talus Spacer is an alternative treatment option to fusion and amputation, and requires talus replacement surgery. During this procedure the native talus bone is removed and replaced with a 3D printed replica. This is considered a joint-sparing procedure, as it allows the patient to maintain motion of his/her ankle joint.
Learn more about Xiomalis and her experience with the Paragon 28 Patient Specific Talus Spacer.
Humanitarian Device. Authorized by Federal law for use in the treatment of avascular necrosis of the ankle joint. The effectiveness of this device for this use has not been demonstrated.